
This black precipitate is silver sulfide. A thick white precipitate of AgCl will quickly form if chloride ion is present. If sulfide ions are present in the solution, as they would be in a solution of sodium sulfide, they will react with the silver ions to produce a black precipitate. A red - orange to deep red color resulting from the formation of the. In the confirmatory test described by the question, silver nitrate is added to a solution of the unknown salt. Then, a confirmatory test is generally performed to further verify the results from the primary test.

This black precipitate is lead(II) sulfide, a product in the reaction between hydrogen sulfide and lead(II) acetate. The presence of hydrogen sulfide and, thus, sulfide ions in the salt is confirmed when a black precipitate is formed on the paper. Approximately 2.0 104 gal nitric acid was spilled. The spill was neutralized with sodium carbonate: 2HNO3 (aq)+Na2CO3 (aq)2NaNO3 (aq)+H2O (l)+CO2 (g) a. The presence of hydrogen sulfide gas is detected using a paper moistened with lead(II) acetate. On Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado. The reaction between the sulfide anions in the salt and the hydrogen cations from the acid produces the foul-smelling gas hydrogen sulfide.

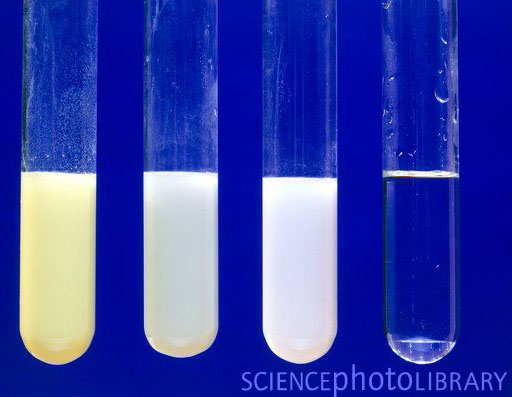
The primary test for sulfide ions in an unknown salt is conducted by first adding a dilute acid, such as hydrochloric acid. What is the color of the precipitate resulting from the formation of Ag2S? (A) White, (B) yellow, (C) brown, or (D) black.Īdding silver nitrate to a solution can be used as a confirmatory test to determine if sulfide ions are present. Plan: Materials: ZnSO4 solution BaCl2 solution AgNO3 solution Pb(NO3)2 solution NaCl solution NaBr solution CuSO4. It is predicted that a solid will form only when there is a change in color. Adding AgNO3 to a Na2S solution forms Ag2S. Furthermore, writing the equations for the reactions and apply the rules of solubility to see if every color change equals to a precipitate.


 0 kommentar(er)
0 kommentar(er)
